Give the Number of Valence Electrons for Xecl4
100 15 ratings Ans valence electron of Xe 8 Va. We will calculate the valence electrons of both these atoms to determine the total number of valence electrons of XeF4.
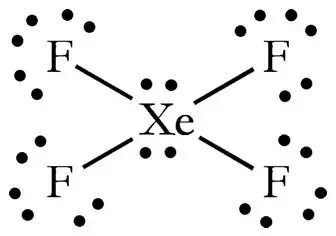
How Can The Lewis Structure For Xef4 Be Determined Quora
Based on the number of valence electrons in a sulfur atom predict the molecular formula of the compound.

. Give the number of valence electrons for SI4. The electron geometry is octahedral while the molecular geometry is square planar Xenon has 6 bonding electron pairs therefore the electron geometry of octahedral but two of the pairs of electrons on the central atom are unbonded or lone pairs therefore the molecular geometry is square planar. To achieve a stable state the p shell needs to accommodate 6 electrons so there is a scarcity of two electrons which makes valence electrons for oxygen 6.
Carbon is surrounded by 4 electron groups. It has 6 protons 6 neutrons and 6 electrons. Give the number of valence electrons for CH2I2.
Carbon-12 is the most common isotope of carbon. Who are the experts. Calculate the total number of valence electrons in the BBr3 molecules outermost valence shell.
Consider the following xenon compounds. XeCl4 CH4 SF4 C2Cl2. Youll want to calculate the formal charges on each atom to make sure you have the best Lewis structure for XeCl 4.
Oxygen is in group 6. Draw lines between S and F to show bonds and for lone pairs of electrons use dots. This Valence Electrons chart table gives the Valence Electrons of all the elements of periodic table.
The atomic number of the oxygen is 8 where its electronic configuration is 1s2 2s2 2p4. The atomic number of the carbon is six which makes its electronic configuration 1s2 2s2 2p2. Each fluorine atoms has nine electrons and there are seven valence electrons in the outer shell of the Bromine molecule out of which three electrons form bonds with three fluorine atoms.
Give the number of valence electrons in each element. Xe 8 valence e-Cl 7 valence e-x 4. The maximum number of valence electrons that can be written around an atom is eight.
Valence electrons of Xenon 8. Each fluorine atom will have three pairs of 6 valence electrons. For neutral atoms the number of valence electrons is equal to the atoms main group number.
Give the number of valence electrons for XeCl4. So now eight valence electrons are used reducing the number of valence electrons from 34 to 24. Sulfur combines with hydrogen by covalent bonding to form a compound hydrogen sulfide.
Then the total outermost valence shell electrons can be calculated as follows. The XeCl4 molecule has one central xenon and four chlorine atoms. All the fluorine atoms have six valence electrons and the central atom has two valence electrons.
The first step is to determine how many electrons are in the BBr3 Lewis structures outermost valence shell. Therefore 74 shall give us 28. Xenon tetrachlorideXeCl4 has the composition of one xenon and three chlorine atoms.
This problem has been solved. Three Cl atoms are bonded to three single electrons and one electron is added to the remaining single electron to give the negative charge on the ion. I XeCl2 ii XeCl4 iii XeO4 iv XeOCl4 v XeO3 Which of the compounds isare polar.
The atomic number of sulfur is 16. Click on Element Atomic Number Element Symbol Element Name and Element Valence Electrons headers to sort. Describe a sigma bond.
Xenon shows the least electronegative property. The main group number for an element can be found from its column on the periodic table. Give the number of valence electrons for XeCl4.
XeF4 Valence electrons. In this molecule we have one atom of Xenon and four atoms of Fluorine. Give the approximate bond angle for a molecule with an octahedral shape.
We review their content and use your feedback to. Thus there are a total of 18 valence electrons available for Ozone molecule. Here as there are three oxygen molecules the total number of valence electrons is 63 18.
Write out the electronic configuration for the valence electrons. O 36 In order to bring out more details and contrast from the shadows and highlights in a black and white image Ansel Adams used the technique of. O3 Valence electrons.
Choose the best Lewis structure for NH4. The Lewis structure for XeCl 4 requires you to place more than 8 valence electrons on Xe. Xenon Xe can have more than 8 valence electrons in your Lewis structure.
Chlorine atoms in a demands electrons. The central Xe atom has 4 bond pairs of electrons and two lone pairs of electrons. A The atomic number of neon is.
An electron in an atoms outermost shell is known as a valence electron. Valence Electrons Chart - Valence Electrons of all the elements in table chart. See the answer See the answer See the answer done loading.
Valence electrons of Fluorine 74 as there are four Fluorine atoms we will multiply it by 4. View the full answer. Asked Aug 2 2019 in Chemistry by Dominican.
In Ozone or O3 there are six valence electrons for each molecule of Oxygen. Step 1 of 3. Total outermost valence shell electrons available for XeCl4 Lewis structure dot structure 847 36 valence electrons in XeCl4.
Give the number of valence electrons for CH2I2. Chlorine and xenon have seven and eight valence electrons respectively. BrF3 is a perfect example of an AX5 molecule with two lone pairs of electrons and three bonded pairs of electrons.
Give the number of valence electrons for XeCl4. Experts are tested by Chegg as specialists in their subject area. Hence there are 36 valence electrons in the compound XeF4.
For example carbon is in group 4 and has 4 valence electrons. Lewis structure of single carbon and oxygen atom separately is as shown below. What is the molecular geometry of xenon tetrachloride.
It is represented by dots in the BBr3 Lewis diagram. XeCl 4 has a total of 36 valence electrons. N single bonded to 4 H 8 total electrons.
The total valence electrons of XeF4 come to be 828 which is 36. BrF3 electron geometry.

Best Overview On Is Xef4 Polar Or Nonpolar Science Education And Tutorials

Best Overview Xecl4 Molecular Geometry Science Education And Tutorials

How To Draw So3 Lewis Structure Science Education And Tutorials

How To Draw Xecl4 Lewis Structure Science Education And Tutorials

Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules

Draw The Lewis Structure For Pcl Then Answer The Following Questions Homeworklib

Solved Give The Number Of Valence Electrons For Xecl4 38 O Chegg Com

How To Draw Xef4 Lewis Structure Science Education And Tutorials
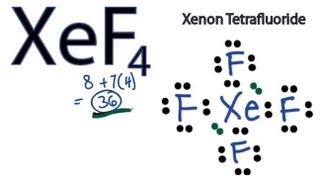
Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube

Draw The Lewis Structure For Xecl4 And Provide The Following Information A Formal Charge For Each Atom B Total Number Of Electron Domains C Electron Geometry D Molecular Geometry E Polarity

How To Calculate The Formal Charges For Xef4 Xenon Tetrafluoride Youtube

Icl3 Molecular Geometry Science Education And Tutorials

Xecl4 Lewis Structure How To Draw The Lewis Structure For Xecl4 Xenon Tetrachloride Youtube

How To Draw Xecl4 Lewis Structure Science Education And Tutorials
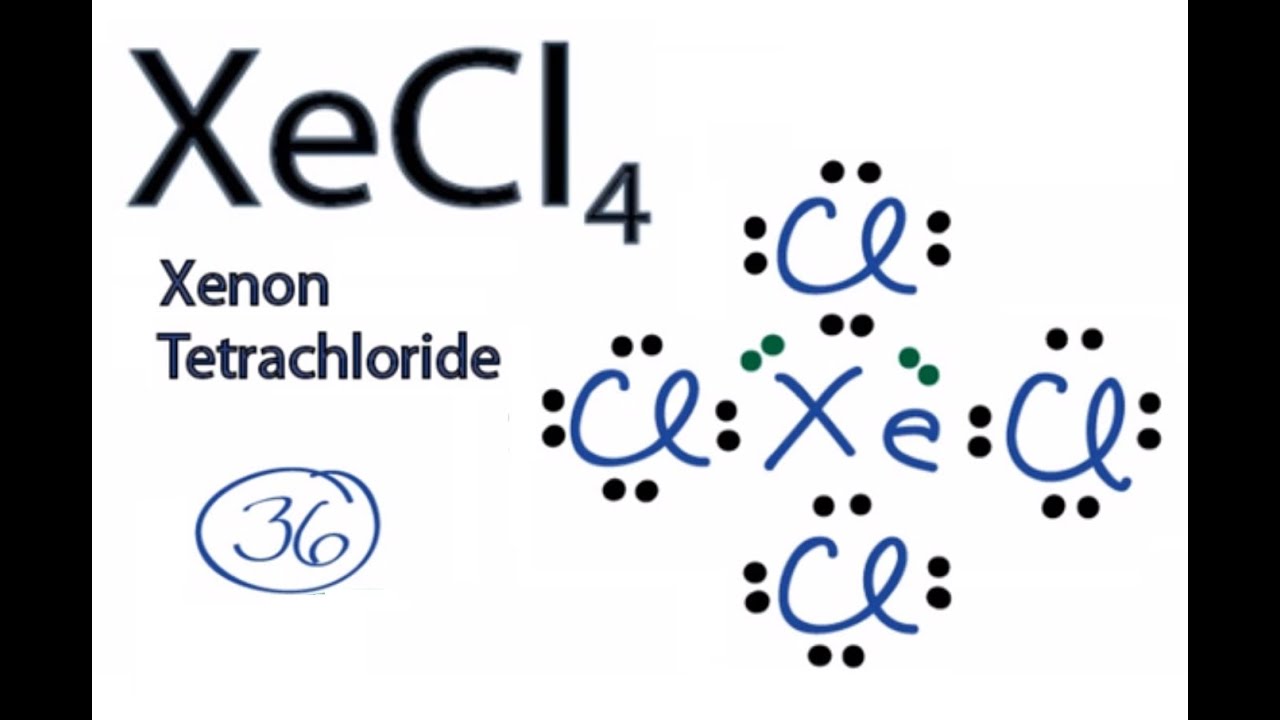
Xecl4 Lewis Structure How To Draw The Lewis Structure For Xecl4 Xenon Tetrachloride Youtube

Clf5 Lewis Structure How To Draw The Lewis Structure For Clf5 Chlorine Pentafluoride Youtube

Xecl4 Lewis Structure How To Draw The Lewis Structure For Xecl4 Xenon Tetrachloride Youtube

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Octet Rule Lewis Noble Gas
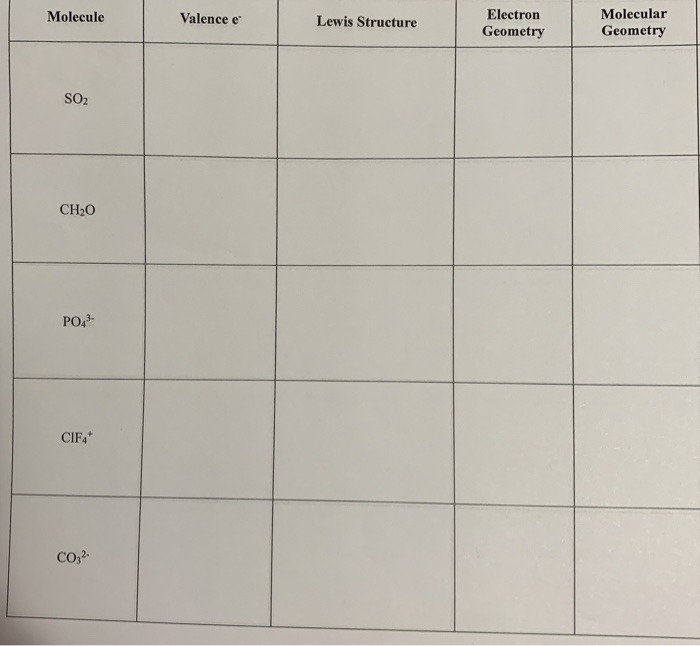
Solved Molecular Geometry Molecule Valence E Lewis Structure Chegg Com
Comments
Post a Comment